Preparation of LiMPS membranes
To synthesize the MPS3 (M = Cd or Mn) crystals, the metal (Aladdin, 99%), red phosphorus (Aladdin, 99.99%) and sulfur (Aladdin, 99.5%) powders were mixed with a molar ratio of 1:1:3 and vacuum sealed in a quartz ampoule, which was then placed in a tube furnace at 700 °C for 7 days13. Every 10 g of MPS3 crystals was washed with 100 ml of ethanol (Aladdin, 99.5%, H2O ≤ 0.005%) 3 times and dried at 60 °C under vacuum for 10 h (environmental chamber, static heating). LiMPS nanosheets were synthesized through a two-step alkali-ion intercalation process13,50. For the LiCdPS, 6.5 g of CdPS3 crystals was added to 10 ml of a mixed aqueous solution of 1 M KCl, 0.5 M K2CO3 and 0.5 M ethylenediaminetetraacetic acid (EDTA) and hydrothermally reacted at 100 °C for 2 h. This process was carried out in a high-pressure autoclave placed in a convection oven under an air atmosphere. This step introduced Cd vacancies by K+ intercalation. The resulting suspensions were washed 3 times with 100 ml of deionized water (with a resistivity of 18.2 MΩ cm at 25 °C) to remove excess K+. Ten millilitres of a 2 M LiCl solution was then added to the washed KCdPS and hydrothermally reacted at 100 °C for 2 h in a high-pressure autoclave placed in a convection oven under an air atmosphere. The products were washed 3 times with 100 ml of deionized water (with a resistivity of 18.2 MΩ cm at 25 °C) to remove excess Li+. The final LiCdPS product was then redispersed in deionized water (with a resistivity of 18.2 MΩ cm at 25 °C) and sonicated for 15 min to promote exfoliation. Sonication was performed using an ultrasonic cleaner (Sunne, SN-QX-32D) at a power of 220 W and 25 °C under an air atmosphere. The LiCdPS dispersions were filtered using a vacuum filtration apparatus (Sunne, SN-SHZ-D) equipped with 0.22 µm polycarbonate membranes as substrates, and the LiCdPS membranes were obtained. LiMnPS membranes were prepared similarly, without K2CO3 and EDTA in the first hydrothermal step.
Preparation of the PA-LiMPS/PEO electrolyte
For the preparation of Li-ion conductive polymeric membrane, PEO (Aladdin, ~600,000 g mol−1, purity ≥98%) and LiTFSI (Aladdin, 99.9%, H2O ≤ 0.005%) were used, without previous drying. The PEO layer was prepared by first dissolving 1 g of PEO and 0.4 g of LiTFSI in 16 ml of acetonitrile (Aladdin, anhydrous, 99.8%) and then stirring for 12 h at 25 °C, using a magnetic stirrer (Joanlab, MMS4Pro) with a Teflon-coated stir bar. The solution was poured into a polytetrafluoroethylene dish (Zhejiang Jusheng Fluorine Chemical, 60 cm × 60 cm) and vacuum dried using a heated vacuum chamber with static heating for 24 h to form solid PEO membranes.
For comparison, other polymer membranes were prepared, including ‘Pure-PEO’ membranes (without LiTFSI salt), ‘PEO-NanoAl2O3’ membranes (LiTFSI replaced with Al2O3 (Aladdin, 99.9%, with an average particle size of 30 nm)) and ‘Pure-PPC’ (poly(propylene carbonate), Aladdin, ~50,000 g mol−1) membranes with no salt additives. All other preparation steps were the same as those used for preparing the PEO membrane. The fabrication of the PA-LiMPS/PEO composite electrolyte membrane was as follows (Supplementary Note 1 and Supplementary Fig. 8). First, large-area individual LiMPS (dimensions, 10 cm × 10 cm; thickness, ~15 µm) and PEO membranes (dimensions, 60 cm × 10 cm; thickness, ~20 µm) were cut (mechanical cutting blade under an ambient air atmosphere) to specific size and manually stacked in a zigzag pattern under ambient air to create a bulk LiMPS/PEO, which was then roll-pressed (JNT, A4) under a pressure of ~0.5 MPa in an ambient air atmosphere, and 5–10 layers of these pressed membranes were combined and further pressed under ~0.5 MPa. This process was repeated until the desired thickness was achieved. The weight ratio of LiMPS and PEO layers was controlled at 55:45. Finally, the roll-pressed bulk composite membrane was cut perpendicular to the LiMPS plane to produce the PA-LiMPS/PEO electrolyte. Specifically, at 25 °C and under an ambient air atmosphere, the bulk LiMPS/PEO was fixed onto the sample holder of a precision mechanical slicer (RWD, Minux S700A), where the cutting process was precisely controlled by a high-accuracy blade and positioning system, ensuring uniform membrane thickness and consistent surface flatness. This process enables the production of PA-LiMPS/PEO electrolyte with controllable thicknesses. For comparison, PA-LiCdPS/Pure-PEO, PA-LiCdPS/PEO-NanoAl2O3 and PA-LiCdPS/Pure-PPC electrolytes were prepared, using Pure-PEO, PEO-NanoAl2O3 and Pure-PPC as the polymer, respectively. In the verification of spray-coating method, ultrasonic spray coating technology was used to directly deposit LiMnPS nanosheets onto a large-area (~20 cm × 20 cm) PEO membrane containing LiTFSI. The entire spray-coating process was carried out using a Cheersonic UAM4000S system, at 25 °C and under an ambient air atmosphere.
Physicochemical characterizations
The morphology and composition characterizations of the LiMPS nanosheets and PA-LiMPS/polymer electrolytes were conducted by a field-emission SEM (TESCAN GAIA3, 2016 UHR)51, a transmission electron microscope (TEM; ThermoFisher Themis Z) at 300 kV and an atomic force microscopy (AFM; Shimadzu SPM9700). TOF-SIMS was performed using a pulsed 30 keV Ga+ beam on the TESCAN GAIA3 system, and a current of 5 nA (ref. 51). The 7Li ssNMR spectra were recorded on a Bruker AVANCE 600 MHz spectrometer. WAXS was performed using a Rigaku HomeLab instrument with a CuKα X-ray beam, and the scattering signal was distributed in a mode perpendicular to the stretching direction. The Hermans orientation factor f was calculated using the azimuthal angle plots obtained from the 2D WAXS images using (equation (1)) and (equation (2))52:
$$f=\frac{\,3\langle {\cos }^{2}\varphi \rangle -1}{2}$$
(1)
$${\cos }^{2}\varphi =\frac{{\int }_{0}^{\frac{{{\uppi }}}{2}\,}I\left(\varphi \right){\,\cos }^{2}\varphi \sin \varphi {\rm{d}}\varphi \,}{{\int }_{0}^{\frac{{{\uppi }}}{2}\,}I\left(\varphi \right)\sin \varphi {\rm{d}}\varphi \,}\varphi$$
(2)
where φ denotes the azimuthal angle and I(φ) denotes the scattering intensity. 〈cos2φ〉 was obtained by azimuthal integration of the selected diffraction peak52. f values range from −0.5 (perfect alignment) to 0 (isotropic structure).
Thermogravimetric analysis (TA Q50 analyser) was performed under nitrogen at 10 °C min−1. Nanoindentation tests (Hysitron TI 950 TriboIndenter) were carried out at 25 °C. Tensile measurements were conducted on a universal testing machine (model 2203) at 25 °C. For the air stability measurements, 100 mg of PA-LiMPS/PEO electrolyte was placed in a sealed 2 l chamber with an H2S gas detector (GX-2009, Riken Keiki). The container was filled with air at 30–40% relative humidity, and the tests were conducted at 25 °C, in a constant temperature environmental chamber (QAS100-T2) with static heating. After cycling, the cells were disassembled inside an Ar-filled glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm, 25 °C). For the collection, the electrolyte membranes were removed from the electrodes using plastic tweezers to avoid mechanical damage. For the collection, the electrolyte membranes were cut to the desired dimensions inside the glovebox, depending on the requirements of the subsequent characterization. For all the physicochemical characterizations carried out ex situ, the cycled electrolyte membranes were transported from the glovebox to the specific instrument without requiring a sample holder with an inert atmosphere. The exposure of the electrolyte to air was controlled within 2 min.
Electrochemical characterizations
Electrochemical impedance spectroscopy (EIS) tests were performed on a CHI 760E workstation (Chenhua), using 1 potentiostatic over 1 MHz to 0.01 Hz with an amplitude of 10 mV, and 12 points per decade. The electrolytes were sandwiched between 2 stainless-steel spacers (Canrd, 99.9% purity, with a diameter of 15.8 mm and a thickness of 1 mm) and assembled in a 2025-type coin cell configuration (Canrd). A pressure of 0.025 tonne was applied during crimping. The ionic conductivity (σ) of the electrolytes was calculated using (equation (3))
$$\sigma =\frac{L}{{S\; R}}$$
(3)
where L is the distance between two blocking electrodes (that is, the stainless-steel spacers), S is the effective area of the electrolyte membrane and R is calculated from the Nyquist plot of the EIS raw data at the intercept with the x-axis.
To measure the in-plane ionic conductivity of LiMPS nanosheets, the free-standing LiMPS membrane was cut into rectangular strips (mechanical cutting blade (for example, Deli) at 25 °C under ambient air) with lateral dimensions of 5 cm × 1 cm and a thickness of 15 µm. Both ends of the membrane were then attached to copper wires using conductive silver paste (Alfa Aesar, ~0.05 g per electrode) to ensure good mechanical and electrical contact (Supplementary Fig. 3). Copper wires (Alfa Aesar, 99.99% purity) with a diameter of 0.5 mm and a length of 10 cm were used. Before electrochemical measurements, the wires were cleaned by ethanol (Aladdin, 99.5%, H2O ≤ 0.005%), followed by drying under ambient air. The in-plane ionic conductivity was calculated using equation (3), where L is the distance between two blocking electrodes (that is, the copper wires), S is the effective area (multiplying the thickness by the width of the membrane) and R is calculated from the Nyquist plot of the EIS raw data at the intercept with the x-axis. The EIS measurements were conducted on three independent samples at each temperature, and the ionic conductivity values shown in the figures represent the average across multiple cells. The EIS measurements at different temperatures were all conducted in a temperature-controlled environmental chamber at static heating, with a temperature accuracy of ±0.1 °C. The assembled cells were rested at open circuit potential for 12 h before EIS measurements to allow the system to reach a quasi-stationary potential state and ensure reliable impedance measurements.
The activation energy (Ea) was calculated from the Arrhenius equation (equation (4)):
$$\mathrm{ln}(\sigma T)=\mathrm{ln}{\sigma }_{0}-\frac{{E}_{{\rm{a}}}}{{RT}}$$
(4)
where σ0 is the pre-exponential factor.
Linear sweep voltammetry was performed over a potential range of 2.0–7.0 V at 10 mV s−1, using electrolyte placed between a Li metal foil (Canrd, 99.9% purity, 50 μm thick, 10 mm diameter) and a stainless-steel spacer (Canrd, 99.9% purity, with a diameter of 15.8 mm and a thickness of 1 mm) in a 2025-type coin cell configuration (Canrd). A pressure of 0.025 tonne was applied during crimping. Using the same cell components, symmetric Li||Li 2025-type coin cells were assembled to investigate the galvanostatic cycling stability of electrolytes, and were tested at current densities of 0.1 mA cm−2, 0.5 mA cm−2 and 3 mA cm−2 with corresponding areal capacities of 0.1 mA h cm−2, 0.5 mA h cm−2 and 3 mA h cm−2 at 25 °C in an environmental chamber, using a Land battery testing system (CT3002A). The critical current density test was conducted by assembling symmetric Li||Li 2025-type coin cells (same component specifications and assembly conditions as above) with various electrolytes and cycling at current densities in the 0.02−10 mA cm−2 range. Using the PA-LiMPS/PEO electrolyte, 6Liǀǀ6Li 2025-type coin cells were assembled (same component specifications and assembly conditions as above, except for the 6Li from Alfa Aesar, 99.9% purity, 200 μm thick, 10 mm diameter) and tested to obtain electrolyte samples for the ex situ TOF-SIMS and ssNMR measurements.
Fabrication and battery testing of the all-solid-state lithium coin and pouch cells
The LiFePO4 and LiNi0.8Co0.1Mn0.1O2 positive electrodes were prepared by mixing (with a mortar and a pestle, mixing for 10 min under ambient air) LiFePO4 (Canrd, average particle size ~2 µm, carbon-coated, ≥99% purity, dried at 120 °C for 12 h before use) or LiNi0.8Co0.1Mn0.1O2 (Canrd, average particle size ~5 µm, carbon-coated, ≥99% purity, dried at 120 °C for 12 h before use), Super P (Canrd, average particle size ~40 nm, ≥99% purity) carbon additive, polyvinylidene difluoride (PVDF) (Canrd, ≥99% purity) and LiTFSI (Aladdin, 99.9%, H2O ≤ 0.005%), without previous drying, in weight ratios of 75:10:10:5 in N-methyl-2-pyrrolidone (Aladdin, 99.5%), followed by casting the slurry (automatic coating machine) on an Al foil (Canrd, 20 µm thick, 15 cm × 15 cm, used as received). LiTFSI is incorporated to form continuous ion conduction pathways between electrode particles. After drying at 100 °C for 12 h (under vacuum (~0.1 MPa) in an environmental chamber with static heating), the composite positive electrodes were prepared with a mass loading of 1.5–2.0 mg cm−2 (active material), having a diameter of 8 mm and a thickness of 40 µm. The electrodes were cut using a mechanical cutting blade at 25 °C under ambient air. The composite positive electrodes were stored in an Ar-filled glovebox (MIKROUNA, O2 < 0.1 ppm, H2O < 0.1 ppm) for subsequent cell assembly. With the PA-LiMPS/PEO or RA-LiMPS/PEO electrolyte, all-solid-state Li||LiFePO4 and Li||LiNi0.8Co0.1Mn0.1O2 2025-type coin cells were assembled in an Ar-filled glovebox (MIKROUNA, O2 < 0.1 ppm, H2O < 0.1 ppm). The pouch cells were assembled in an Ar-filled glovebox (MIKROUNA, O2 < 0.1 ppm, H2O < 0.1 ppm), with a layer of Li metal negative electrode, a layer of PA-LiMPS/PEO electrolyte (3.9 cm × 3.9 cm, 200 µm thick) and a layer of LiFePO4-based positive electrode. The size of the pouch cells was 4 cm × 4 cm. The Li||LiFePO4 all-solid-state batteries (pouch and coin formats) were tested in the current density range 0.034−1.7 mA cm−2. The Li||LiNi0.8Co0.1Mn0.1O2 all-solid-state batteries (coin format) were tested in the current density range 0.04–2.0 mA cm−2. The force applied during coin cell assembly was set to 0.025 tonne, resulting in a stack pressure of less than 0.5 MPa. For pouch cells, the assembly and sealing were carried out with a packaging pressure of less than 0.1 MPa. During electrochemical testing and cycling, no external pressure was applied, using a Land battery testing system (CT3002A). The specific capacity values in the figures refer to the mass of the active material in the composite positive electrode. The battery tests were conducted in a temperature-controlled environmental chamber with static heating, maintaining an average temperature of 25 °C with a precision of ±0.1 °C.
Density functional theory calculations
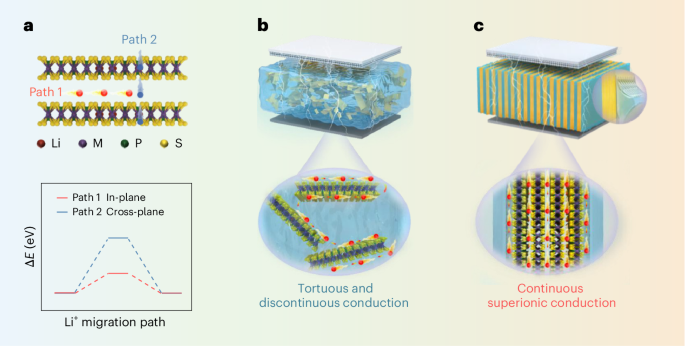
Density functional theory (DFT) calculations were conducted using the Vienna Ab initio Simulation Package. The general gradient approximation functional of Kresse et al.53 and the projector augmented-wave method54 were used to describe core–valence interaction. A plane-wave cut-off energy of 400 eV was used, with convergence thresholds of 1 × 10−8 eV for energy and 0.02 eV Å−1 for forces. A vacuum layer exceeding 15 Å prevented interactions between periodic images. Using DFT-D355, van der Waals interactions were corrected. A 3 × 3 × 1 k-point mesh was applied for geometry optimization and nudged elastic band calculations. Initial and final atomic configurations for in-plane and cross-plane Li migration barriers are shown in Supplementary Fig. 1. Nudged elastic band calculations used six intermediate images.
COMSOL numerical analysis
COMSOL numerical analysis was used to simulate the ion current flow in the PA-LiCdPS/PEO and RA-LiCdPS/PEO composite electrolytes, with PEO containing LiTFSI. Two specific models were constructed for analysis: (i) PEO layers alternating with perpendicularly aligned LiCdPS layers and (ii) PEO with randomly distributed LiCdPS nanosheets. The conductivity of the PEO was set at 5.4 × 10−3 mS cm−1 based on experimental measurements. The in-plane and cross-plane ionic conductivities of LiCdPS were respectively set at 120 mS cm−1 and 6.5 × 10−3 mS cm−1 at 25 °C, based on experimental measurements. The direct current conduction mode of COMSOL Multiphysics was used, applying a constant input potential of 1 V at the top surface with the bottom surface grounded to analyse the current density distribution within the composite structures. The simulation results allowed for the calculation of the effective conductivities of the PA-LiCdPS/PEO and RA-LiCdPS/PEO composites, which were determined by averaging the current density at the ground and given input potential.